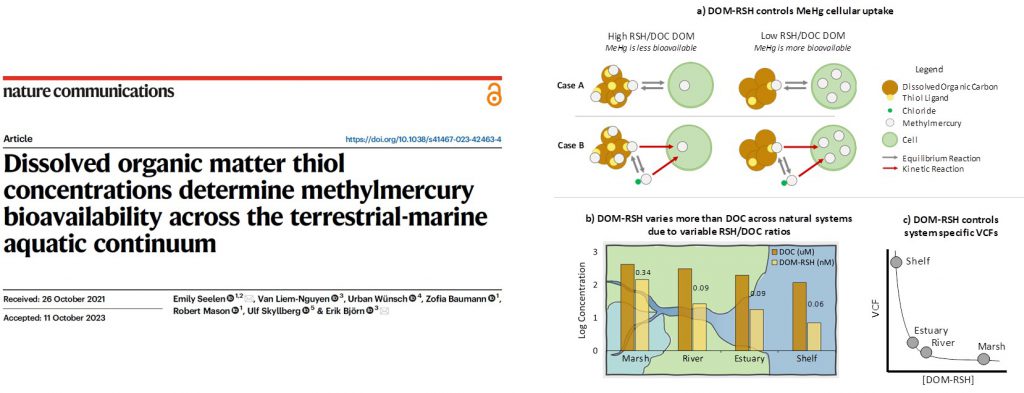
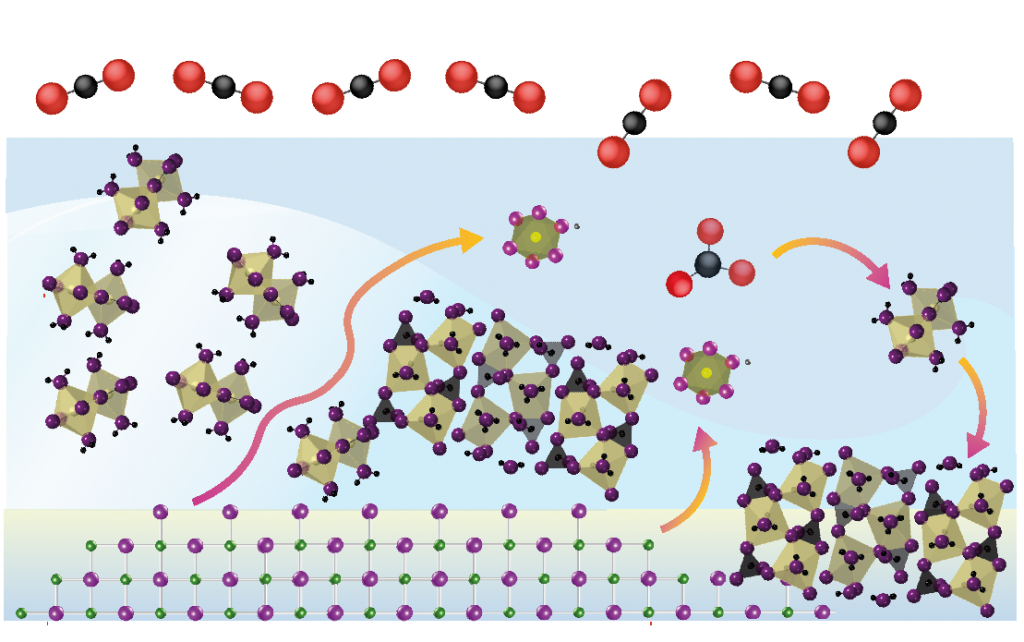
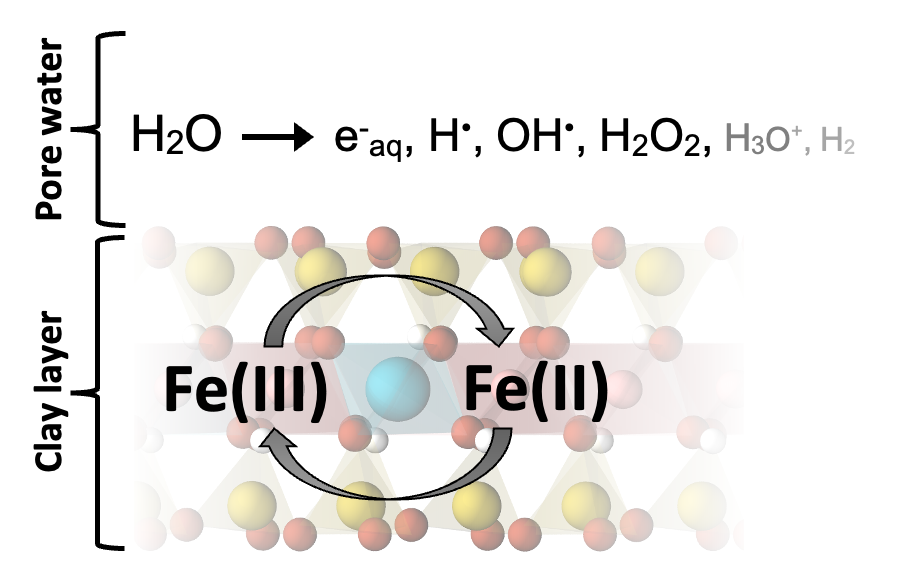If chemistry is the central science, then geochemistry is the central science as applied to understanding the natural world around us.
If chemistry is the central science, then geochemistry is the central science as applied to understanding the natural world around us.
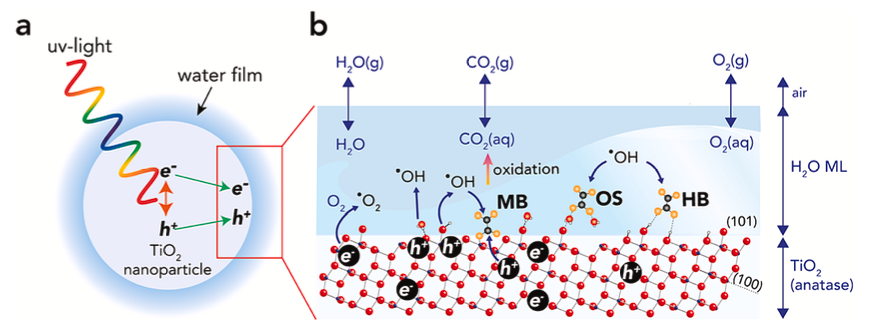
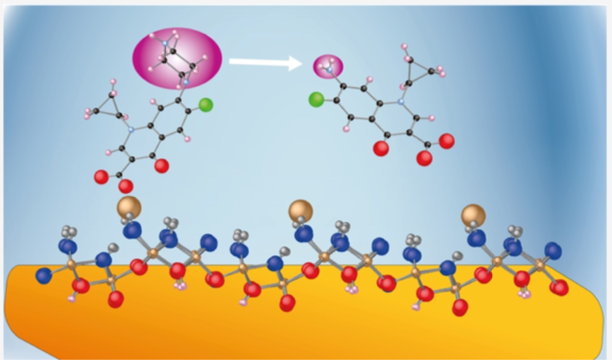
Geochemists seek to answer questions relating to the evolution of life on Earth and how metalloenzymes may have evolved, the chemistry of the oceans and how they are affected by global warming, the interplay between flora, fauna and the environment in chemical terms, how pollutants interact with soils and minerals, and how radioactive waste can be securely stored for millennia. We do this by connecting the very big — mountains — with the very small — atoms and molecules, and the very fast — fundamental reactions — with the often very slow — weathering
If you share our passion for understanding and explaining how the world works — join us! To find out about opportunities in our laboratory, contact one of the group leaders: Erik Björn, Jean-François Boily, Michael Holmboe, C. André Ohlin, Andrey Shchukarev, and Staffan Sjöberg.